Darwin's God of Time is a chimera.
$cientism and the religion of deep time. Some basic analysis reveals the poverty of the claim of 'billions of years'.
Précis
Darwinism and the religio-poli-cracy of Evolution relies on having faith in the God of Time to take nothing to everything. Microbiology, irreducible complexity, mathematical probability and real science destroy the facile Victorian theology that random chance can generate species transmutation and metamorphosis. No evidence for this exists. Evolution also has nothing to say about the origins of life, or arrival of life forms including the arrival of the ‘fittest’.
Given that real science and maths destroy the simplistic mechanical-materialistic view of Darwinians, they fall back on the God of time. If the universe is 13 billion years old, and the Earth some 4 billion, perhaps the Darwinian faithful intone, given various ‘quantum fluctuations’ merged with electromagnetic energy, suffused with chance and experimentation, the variegated life forms on Earth could have formed.
Is the Darwinian belief in long ages really supported by ‘The Science’? The evidence is certainly unkind to the religion of Evolution.
Even if you believe the cosmos is 4 Trillion-Brazilian years old, there is no mathematical probability that your ~70 trillion cells, with circa 6 feet of DNA coding per cell, or 67 billion miles of DNA per person, formed by chance within even that limitless amount of time. Time is a chimera when it comes to the secular religion of materialism. ‘Time’ can never create the conditions to allow the building of functional and coded design that runs for 67 billion miles in the case of a single human’s DNA, not to mention the endless complexities of flora and fauna species. Yet the ‘long ages’ is all Darwinians have when it comes to their holy Trinity of time, mutations, and chance and even those claims are more propaganda than scientific.
Let’s have a look at long-age ‘dating techniques’.
Radio Ga Ga
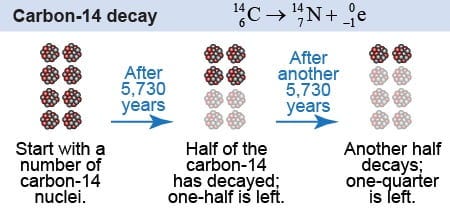
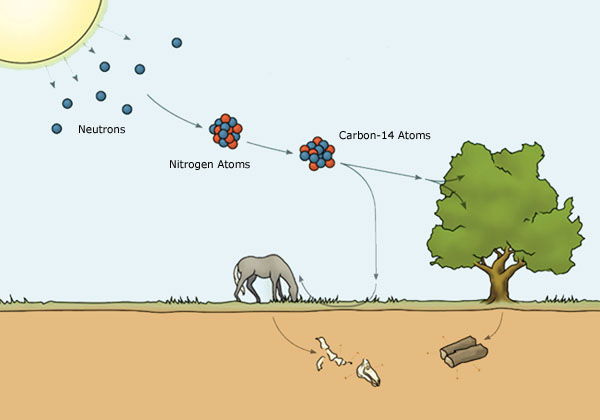
Carbon 14 (C14) is often used to prove long ages, yet carbon 14 radioactive dating is fraught with difficulty and inaccuracy. Such ‘concerns’ are rarely if ever expressed by ‘The Science’, given that they would invalidate the long-age thesis.
C14 is used to date objects containing carbon and has a half-life of 5700 years. This method was developed in 1947 by the American William Libby. He tried to prove that C14 formation in the upper atmosphere is always constant, namely that the rate of C14 creation does not vary. From this assumption we can calculate the carbon 14 in objects on Earth and derive an age.
Libby’s assertion that the base supply of carbon 14 is constant, is of course a ridiculous assumption. There are cosmic, solar and gamma ray events within our solar system on a weekly if not a daily basis. Carbon 14 levels in our upper atmosphere will not be constant and will vary widely depending on these events. It is impossible to assert that the base denominator used in C14 calculations is a never-changing constant. This is simply a facile assumption, not a proven scientific fact.
Richard Lingenfelter corrected Libby and wrote that: 'There is strong indication, despite the large errors, that the present natural production rate exceeds the natural decay rate by as much as 25 per cent. It appears that equilibrium in the production and decay of carbon 14 may not be maintained in detail' (Lingenfelter, p. 51). This is now known to be factual. Even NASA admits to great variations in carbon 14 creation. C14 calculations based on Libby’s assumption are therefore incorrect and wildly inflate object life spans.
Melvin Cook, Professor of Metallurgy at Utah University, reviewed the data of Lingenfelter and many others and reached the conclusion that the present rate of formation of carbon 14 is 18.4 atoms per gram per minute and the rate of decay 13.3 atoms per gram per minute, a ratio indicating that formation exceeds decay by some 38 per cent. This is a massive discrepancy that reduces billions of years down to millions or thousands.
If you apply Suess, Lingenfelter and Cook’s analysis to our atmosphere and go back and use Libby’s own calculations, we get an age of our atmosphere to be roughly, 10.000 years. But don’t tell anyone.
As I wrote before, we also have the inconvenient truth about the formation of coal and diamonds and the problem of DNA from dragon or dinosaur tissue. These all contain carbon and perforce also destroy long ages. A diamond can never be billions of years in age when it possesses carbon 14 with a half-life of 5700 years. The process of making coal is from catastrophe, not from a tree or a dead animal falling in the marsh and being buried over endless time from sediment accretion. Coal has to be formed quickly, from carbon sources tortured under extreme pressure. Dragon or dinosaur soft tissue and DNA is also very unstable and cannot last millions of years. These DNA samples can only be thousands of years old.
So much for carbon 14.
Iso-trope-ing
Given that carbon 14 does not help the long-agers they will turn to rocks. In dating rocks, parent elements are used and compared to daughter element patterns over time. For example, potassium-40 decays to argon-40, uranium-238 decays to lead-206 via other elements like radium, uranium-235 decays to lead-207, rubidium-87 decays to strontium-87 etc. These processes of degradation are applied to igneous rocks to give a time of ‘birth’, dated back to the solidification of its material, or its supposed original age of formation. It is highly conjectural (Hammond et al, 1990).
Isotope concentrations can indeed be measured quite accurately, but isotope concentrations are not dates or a time clock. The two are completely different concepts. To derive ages from such measurements, unprovable assumptions are forwarded:
1. Decay rates have always been constant (this can only be true if unchanging processes are assumed, and rather singular experiments ‘proving’ the decay rate are simply accepted as fact).
2. The initial conditions are known (for example, that there was no daughter isotope present at the start, or that we know the quantity which was originally present). No one has any idea what the starting conditions and metrics are.
3. Systems are closed or isolated so that no parent or daughter isotopes were lost or added (again based on uniformitarian assumptions and false). The Earth is an open system, so too is its geology.
None of the above are truly valid hypotheses and are largely incorrect making isotopic testing unscientific. Massive dating errors prove this. Examples of this include recent volcanic eruptions when rocks are created, thus beginning the dating of igneous rock formations, often give billions of years in age, when the rocks were just recently formed (Austen, 1994).
Isochrony chaos
The isochrony technique involves collecting several rock samples from different parts of the rock unit being dated. It’s methodology is fairly straight forward:
· The concentration of a parent radioactive isotope is graphed against the concentration of a daughter isotope, for all the samples.
· A line is graphed which represents the ratio of parent to daughter. From this a ‘date’ is estimated.
An example would be rubidium-87 graphed against strontium-87 a daughter isotope, comparing the ratio to a stable isotope of strontium-86.
Let’s assume there is drilling into deep granite rock. Within the drill, crystals or ‘zircons’ will be obtained, and these contain uranium which has partly degraded into lead. By measuring the amount of uranium and ‘radiogenic lead’ in these zircon crystals, the theory is that we can calculate age. If the decay rate of U to Pb has been constant, the geological age of a granite sample should be in a range from 125 million to 1.5 billion years, which is the ‘consensus’ age of the Earth’s granite. However:
Helium retention in zircon crystals from the RATE project, much derided by the establishment, found that when uranium decays to lead, a by-product of this decay is the formation of helium, a very light, inert gas, which readily escapes from rock.
Within these zircons, there should not be any helium left, if the age of the rock was millions or billions of years old.
Various labs have confirmed that the helium does indeed seep out very quickly over a wide range of temperatures. In fact, results show that because of all the helium still in the zircons, these crystals (and since this is Precambrian ‘basement’ granite, by implication the whole earth) could not be older than 14,000 years.
There are many other scientific objections to the isochrony chaos, but don’t tell anyone or you will be censored, fired, attacked, calumnied and probably disemboweled.
An example would be the marine fossils found on Everest, the young ages of most mountains and the fact that mountains, canyons and cliffs and other impressive formations must be developed from a catastrophe, not from endless time and uniformitarian depositions or subtle changes due to rivers or rainfall (Larson et al 1994).
Bottom Line
Blindly accepting a ‘postulate’ or ‘law’ is a common failure with ‘The Science’, as given in many posts on this substack (see Einstein, or Copernicanism for example). It is a mixture of fraud, laziness and corruption. Real science always needs to first verify existing assumptions and conventions. Carbon 14, isotope and isochrony testing are examples of this fact. It is frankly inane that subjective testing processes, fraught with unconfirmed postures and convictions, is taught as settled science.
Whatever your worldview is, long age dating is a mess and unsettled. To the objective observer and to anyone pursuing real science, the rather facile and simplistic tools around dating have major issues and need replacing given that mechanical experimentation does not support the existing covenants nor claims.
It is obvious however, that for the gatekeepers of materialism and mechanical science, these rather fraudulent tests are foundational for the maintenance of secular gospel, itself entirely reliant on endless long ages. One can only hope that eventually real science will triumph over the Evolution-Long Ages-military-industrial complex.
Some Sources you should never read
Cook, Melvin A. Prehistory and Earth Models, University of Utah, 1966
Lingenfelter, Richard E. "Production of Carbon-14 by Cosmic Ray Neutrons," Reviews of Geophysics, Vol. 1, No. 1,' February, 1963
McKee, E. H. and D. C. Noble, 1974. Rb-Sr age of the Cardenas Lavas, Grand Canyon, Arizona. In, Karlstrom, T. N. V., G. A. Swann, and R. L. Eastwood (eds.), Geology of northern Arizona, pp. 87–96. Geological Society of America, Rocky Mountain Sectional Meeting, Flagstaff.
Larson, E. E., P. E. Patterson, and F. E. Mutschler, 1994. Lithology, chemistry, age and origin of the Proterozoic Cardenas Basalt, Grand Canyon, Arizona. Precambrian Research 65:255–276.
Hammond, J. G. and J. L. Wooden, 1990. Isotopic constraints on the petrogenesis of Proterozoic diabase in Southwestern USA. In, Parker, A. J., P. D. Rickwood, and D. H. Tucker (eds.), Mafic dykes and emplacement mechanisms, pp. 145–156. Rotterdam: Balkema.
Heaman, L. M. and J. P. Grotzinger, 1992. 1.08 Ga diabase sills in the Pahrump Group, California: Implications for development of the Cordilleran Miogeocline. Geology 20:637–640.
Howard, K. A., 1991. Intrusion of horizontal dikes: Tectonic significance of Middle Proterozoic diabase sheets widespread in the upper crust of the Southwestern United States. Journal of Geophysical Research 96:12,461–12,478.
Austin, S. A., 1994. Are Grand Canyon rocks one billion years old? In, Austin, S. A. (ed.), Grand Canyon: Monument to catastrophe, pp. 111–131. Santee, California: Institute for Creation Research.










Excellent, as usual, thanks. Great book list. Seems to me the covid madness has really begun to get people questioning science. Good.